[ad_1]
4 several types of eyedrops bought at Walmart and CVS have been recalled as a result of officers say there’s a lack of sterility assurance on the facility the place the drops are made. The eyedrops are produced by India-based Brassica Pharma Pvt. Ltd. and distributed nationwide at CVS, Walmart and AACE Prescription drugs Inc.
The circumstances of the ability had been found throughout an inspection by the Meals and Drug Administration.
“These merchandise are supposed to be sterile. Ophthalmic drug merchandise pose a possible heightened danger of hurt to customers as a result of medication utilized to the eyes bypass among the physique’s pure defenses,” reads a portion of the discharge asserting the recall.
The recalled merchandise shouldn’t be utilized by anybody and must be returned to the retailer the place they had been bought. Those that use the eyedrops are liable to potential eye infections or associated hurt, in response to the discharge by the FDA.
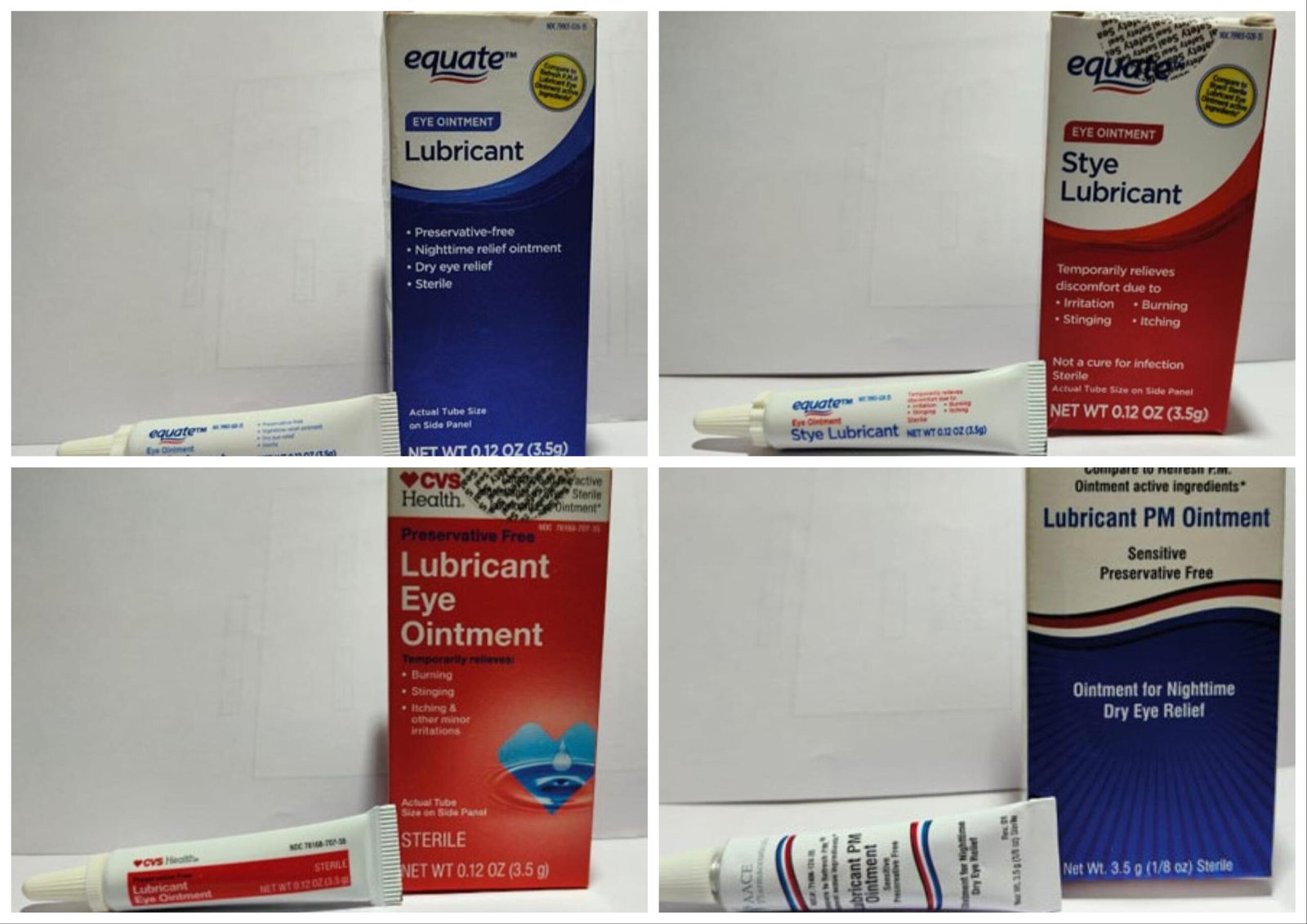
The eyedrops are in 3.5 ounce bottles with expiration dates starting from February 2024 to September 2025. The next model names and their UPC codes have been recalled:
- CVS Well being Lubricant Eye Ointment UPC 050428634141
- Lubricant PM Ointment UPC 371406124356
- Equate Lubricant Eye Ointment UPC Code 681131395298
- Equate Model Lubricant Eye Ointment UPC 681131395304
Thus far, Brassica Pharma says it’s not conscious of any “adversarial occasions” associated to the eyedrops. For extra data on the recall, visit the FDA website.