[ad_1]
4 eye ointment merchandise offered in some Walmart and CVS shops have been recalled as a result of the power during which they have been manufactured in India could have been unsanitary.
In a recall announcement posted Monday on the Meals and Drug Administration web site, Brassica Pharma Pvt. Ltd., a product improvement and manufacturing firm within the Indian state of Maharashtra, mentioned an FDA inspection had famous a “lack of sterility assurance on the facility.”
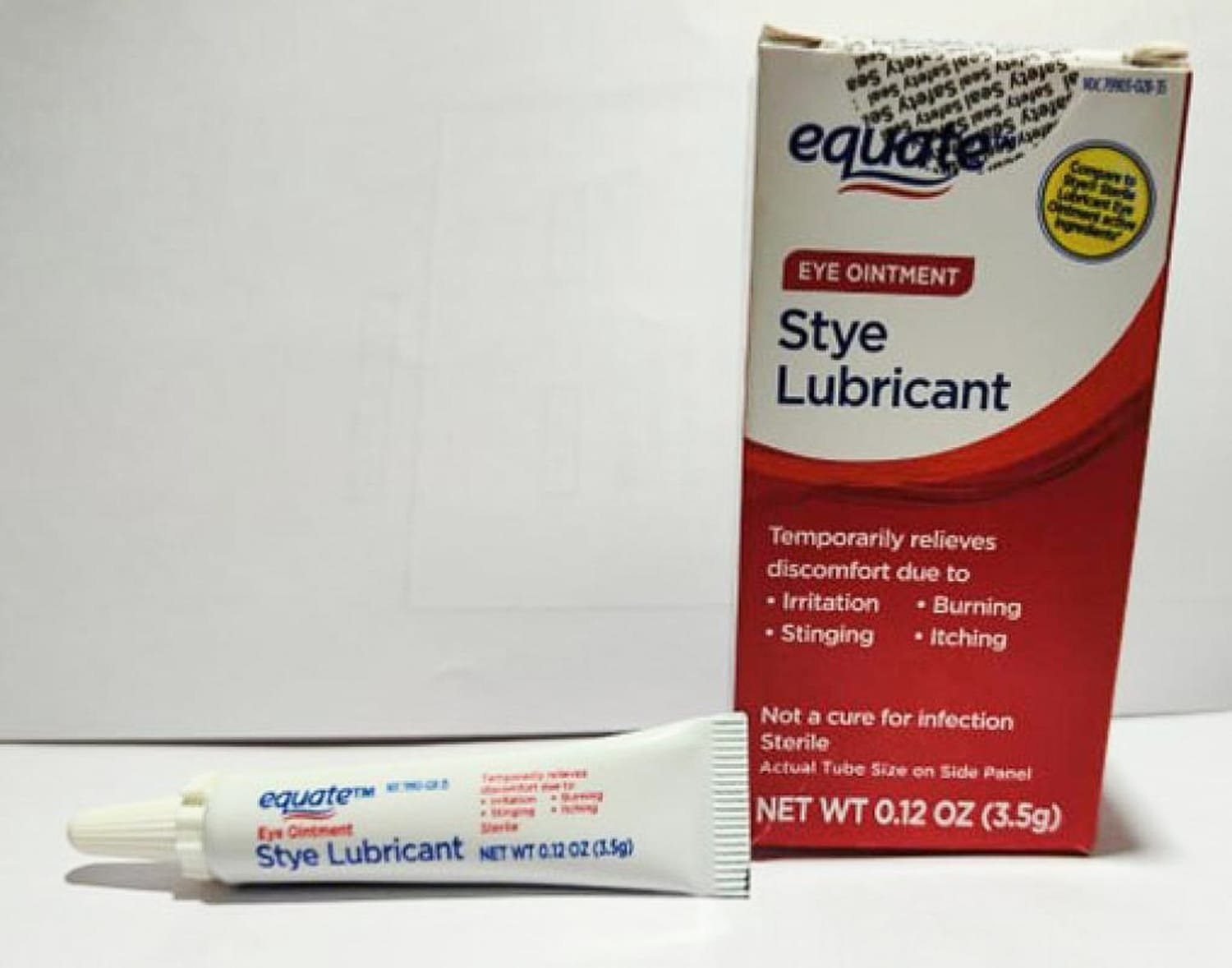
The affected merchandise embody two ointments offered underneath the Equate model title — Lubricant Eye Ointment and Stye Lubricant Eye Ointment — in addition to CVS Well being Lubricant Eye Ointment and AACE Prescription drugs Lubricant PM Ointment.
“For these sufferers who use these merchandise, there’s a potential threat of eye infections or associated hurt. These merchandise are supposed to be sterile,” the announcement mentioned. “Ophthalmic drug merchandise pose a possible heightened threat of hurt to customers as a result of medication utilized to the eyes bypass among the physique’s pure defenses.”
The announcement didn’t specify which sorts of infections customers could also be susceptible to contracting, nor did it give particulars concerning the specific points on the manufacturing facility. Brassica Pharma didn’t reply to requests for additional data or remark.
The recalled merchandise are supposed to alleviate eye dryness or discomfort because of itching, burning, stinging or publicity to wind or solar.
Brassica Pharma had not obtained any studies of an infection or different accidents associated to the recalled ointments as of Feb. 16, in keeping with the recall announcement. However the firm mentioned that buyers ought to cease utilizing the recalled merchandise and that they’ll return them to the shops the place they have been bought.
The recalled merchandise have expiration dates between February 2024 and September 2025, in keeping with the announcement, and are offered in 3.5-gram tubes that come packaged in cardboard containers.
The recall got here after a string of high-profile incidents during which customers contracted severe eye infections from contaminated eyedrops. By May, 81 individuals had been recognized with drug-resistant bacterial infections after having used contaminated eyedrops. Amongst these circumstances, 14 individuals have been blinded, 4 others needed to have their eyeballs surgically eliminated, and 4 individuals died. Many affected sufferers had used drops from the manufacturers EzriCare and Delsam Pharma; these merchandise have been recalled.
In August, the FDA told consumers to not use two different sorts of eyedrops due to a threat of fungal and bacterial contamination. Then the company issued a warning in November about 28 other eyedrop products offered by Goal, Ceremony Assist, CVS and different retailers. A number of of the listed objects, some from the manufacturers Leader, Rugby Laboratories and Kilitch Healthcare India Limited, have since been recalled.
Should you expertise issues after utilizing any of the newly recalled eye ointments, contact your well being care supplier, Brassica Pharma’s announcement says. Folks also can submit studies about issues brought on by the ointments to the FDA’s adverse event reporting program.